joule to erg – How to convert J to erg
The conversion from joule to erg bridges the SI system and the centimeter–gram–second (CGS) system. While the joule is the modern standard for most applications, the erg remains a useful unit in specialized fields like astrophysics and microscopic physics.

What is a joule?
A joule (J) is the SI unit of energy, equal to the work done by a force of one newton moving an object one meter in the direction of the force.
Formula:
1 J = 1 N × 1 m
or
E = ½mv²
It’s used in engineering, physics, chemistry, and daily life — from electricity bills to food energy values.
What is an erg?
An erg is the CGS unit of energy, defined as the work done by a force of one dyne moving an object one centimeter.
Formula:
1 erg = 1 dyne × 1 cm
In SI terms:
1 erg = 10⁻⁷ J
The erg is common in older physics literature, astronomy, and studies where very small energy values are important.
How to convert joule to erg
Because both measure energy, the formula is direct:
Energy (erg) = Energy (J) × 10⁷
Example:
If you have 0.25 J, then
Energy = 0.25 × 10⁷ = 2 500 000 erg
For the reverse conversion:
Energy (J) = Energy (erg) × 10⁻⁷
For quick, precise calculations, try our Energy Converter or explore more unit options with the full Conversion Tools collection.
Do you know?
-
Joule fact: The joule is named after James Prescott Joule, who in the 1840s proved that heat and mechanical work are equivalent, laying the foundation for energy conservation laws.
-
Erg fact: In 1987, astrophysicists calculated that supernova SN 1987A released about 10⁴⁴ erg of energy — a scale that makes joules look cumbersome.
-
Joule fact: One joule equals the energy needed to lift a small apple (about 100 g) one meter against gravity.
-
Erg fact: Before the SI system was adopted, ergs were standard in much of 20th-century physics research, especially in spectroscopy and electromagnetic theory.
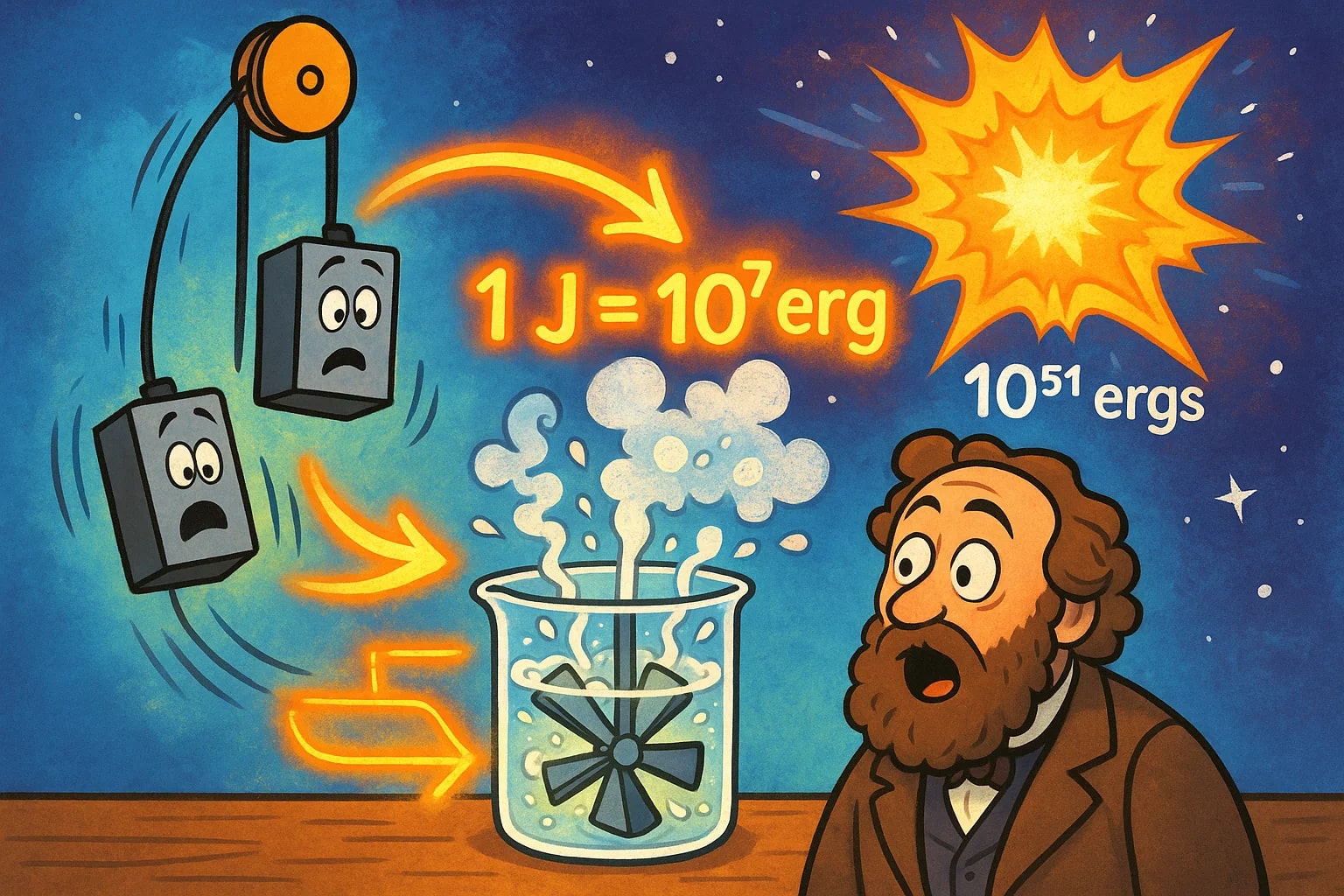
The Experiment That Defined the Link
In the mid-19th century, James Prescott Joule designed a paddle-wheel experiment to prove the mechanical equivalent of heat. He suspended weights over a pulley connected to paddles submerged in water. As the weights descended, they turned the paddles, stirring the water and slightly increasing its temperature.
By carefully measuring the drop distance, the mass of the weights, and the water’s temperature change, Joule determined how much mechanical work produced a given amount of heat. His experiments showed that a specific, consistent quantity of mechanical energy was needed to generate a unit of heat — leading to the definition of the joule.
When the CGS system became popular, scientists needed a smaller unit for precision work. The erg was introduced, perfectly scaled so that 1 J = 10⁷ erg, preserving the exact relationship Joule had established.
This direct scaling made it simple for physicists to move between large-scale SI values and the tiny CGS figures needed for micro-scale or astronomical measurements. Even today, astrophysicists often work in ergs because it keeps the numbers manageable when describing vast energies like those from cosmic explosions.
Speaking the Same Energy Language
The joule to erg conversion is more than moving a decimal point — it’s a bridge between historical and modern systems of measurement. Whether you’re reading century-old research or today’s astrophysics data, being able to move between J and erg ensures clarity and precision.
And while the erg may be less common in everyday life, it remains deeply embedded in scientific literature and practice — a reminder that units evolve, but the energy they measure remains the same.

