kilograms to atomic mass unit – How to convert kg to amu
Ever wondered how to convert kilograms to atomic mass unit? This conversion might sound like comparing apples to atoms, but it’s crucial in fields like nuclear physics, chemistry, and quantum research. In this article, we’ll break down both units, show you how to convert kg to atomic mass unit, and share some fascinating facts that bridge everyday mass with the atomic world.
What is a Kilogram?
The kilogram (kg) is the base unit of mass in the International System of Units (SI). It’s used globally for everything from weighing groceries to launching satellites, and serves as a bridge between macroscopic and microscopic measurements.
Since 2019, the kilogram has been defined using the Planck constant, a breakthrough that aligned it with quantum mechanics.
-
Symbol: kg
-
Defined by: Planck constant (h = 6.62607015 × 10⁻³⁴ Js)
-
Used in: Engineering, physics, logistics, industry
Want to explore more practical conversions like grams, pounds, and tons? Try our Weight Converter collection.
What is an Atomic Mass Unit?
The atomic mass unit (amu), sometimes written as u, is used to express the mass of atoms and molecules. It’s based on the carbon-12 atom: 1 amu is exactly 1/12 the mass of a carbon-12 nucleus. This makes it ideal for describing atomic-scale objects in chemistry, nuclear physics, and quantum mechanics.
-
Symbol: amu or u
-
1 amu = 1.66053906660 × 10⁻²⁷ kg
-
Used in: Particle physics, molecular chemistry, radiophysics
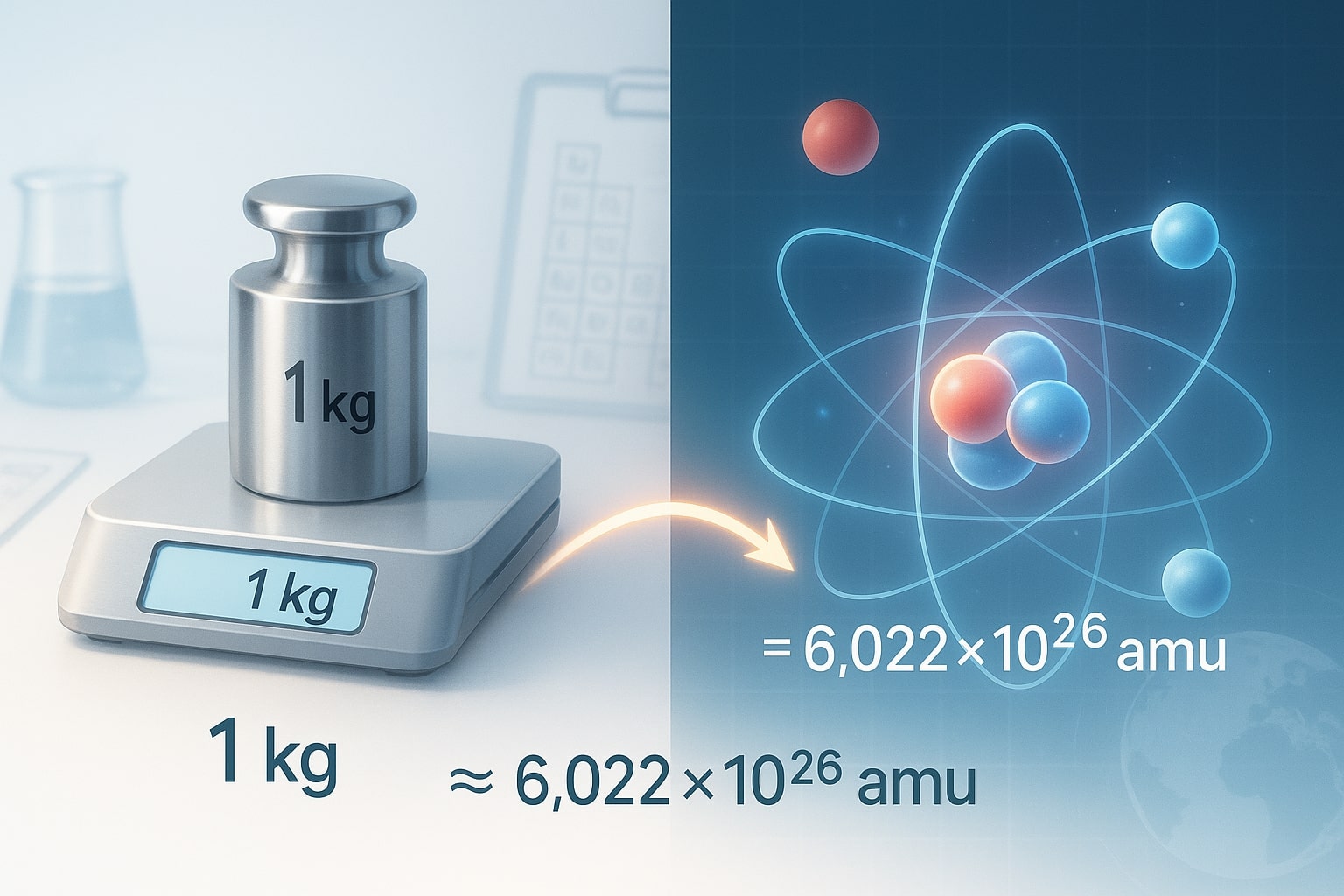
Conversion Formula: Kilograms to Atomic Mass Unit
To convert kg to amu, divide the mass in kilograms by the equivalent of one atomic mass unit in kg:

Example:
1 kg ≈ 6.02214076×1026 amu
This number is directly related to Avogadro’s constant, which defines how many atoms are in a mole. If you'd like a fast result, try our Kilograms to Atomic Mass Unit Converter and skip the math.
Did you know?
-
The kilogram is the only SI base unit that was once defined by a physical object. But after 2019, it was redefined using the Planck constant to ensure long-term consistency.
-
The atomic mass unit is essential for calculating relative molecular masses. For instance, water (H₂O) has a molecular mass of about 18.015 amu, helping chemists balance reactions with atomic-level accuracy.
-
The number of amu in 1 kg (~6.022 × 10²⁶) is the same as Avogadro’s number, connecting everyday mass with moles and atomic quantities in chemistry.
-
According to a 2017 NIST study, redefining the kilogram improved precision in microgravity research and particle mass simulations.
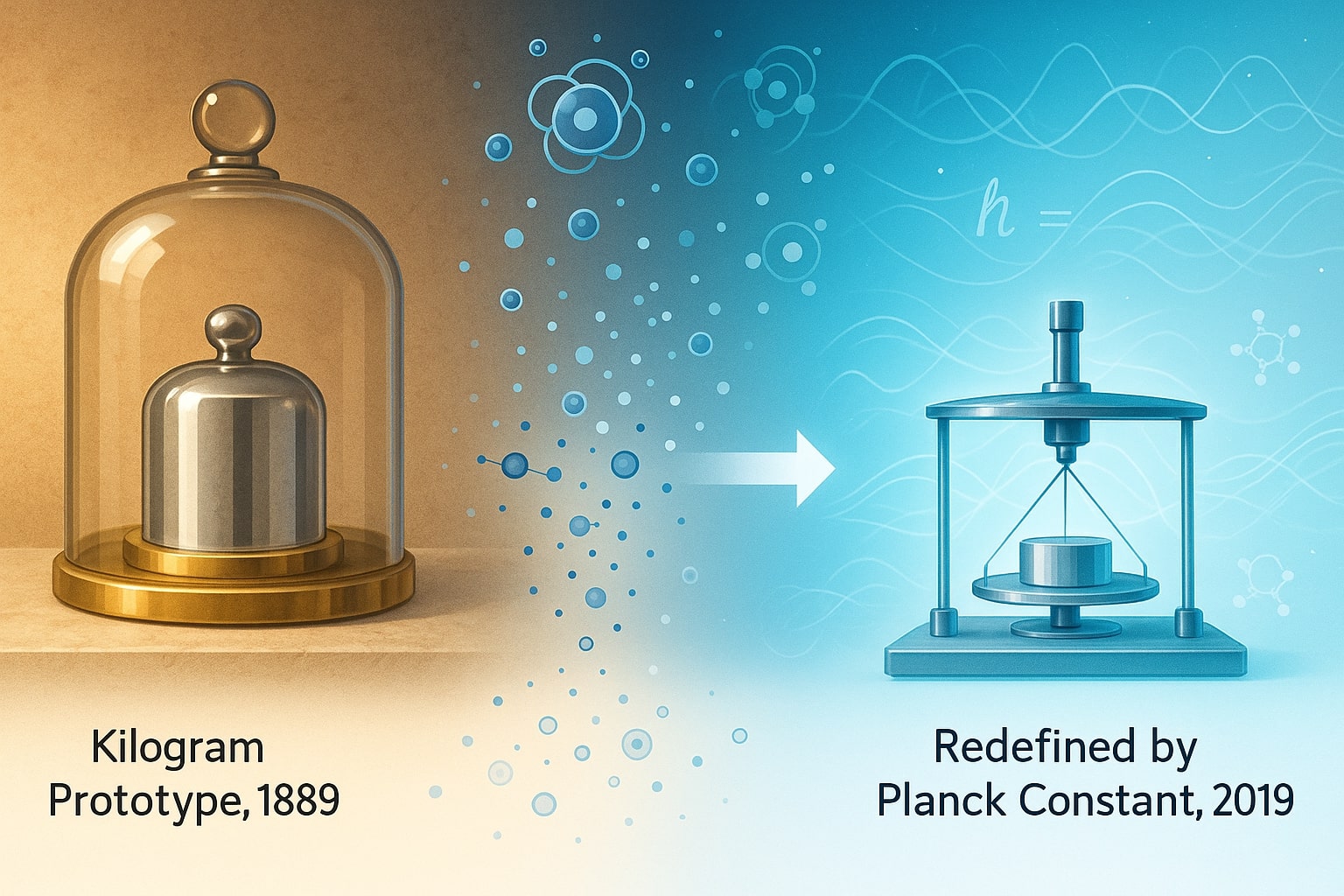
From Platinum Cylinders to Carbon Atoms: The Measurement Revolution
In the late 19th century, the kilogram was represented by a platinum-iridium cylinder stored in a vault in France. But over time, even this ultra-stable object began to lose atoms—making it less reliable as a universal standard.
This led scientists to pursue something more stable: the Planck constant, tied to quantum physics. In 2019, the kilogram was officially redefined by linking it to fundamental constants, just like the atomic mass unit was already tied to carbon atoms.
This shift marked a massive step forward. Now, the kilogram and the amu are both part of a measurement system that spans from the cosmic scale to the atomic, unified by unchanging physical laws.
Conclusion
Converting kilograms to atomic mass unit might seem like scientific trivia, but it’s a bridge between the world you see and the one you can’t. Whether you’re studying chemistry, working in physics, or just curious about how scientists think in atoms and constants, this conversion opens up a new level of understanding.
From shopping bags to subatomic particles, mass matters. And with Jetcalculator, so does precision.
Explore more tools in our Unit Converter or dive deeper with our full range of Weight Converter.

